Background:
γ9δ2 T cells are emerging as a novel tumor-infiltrating effector cell that harbors strong cytolytic and pro-inflammatory activities, and whose intratumoral presence is associated with a favorable prognosis across solid and liquid cancer patients (Gentles et al., 2015; Tosolini et al., 2017). ICT01, a first-in-class anti-BTN3AmAb activating γ9δ2 T cells, completed Phase 1 testing in relapsed/refractory (r/r) solid tumors as monotherapy and in combination with pembrolizumab, and as monotherapy in r/r AML and lymphoma (EVICTION-NCT04243499). ICT01 activates circulating γ9δ2 T, CD8 T, and NK cells that leads to tumor infiltration and remodeling of the TME (De Gassart et al., 2021; Wermke et al., 2021) without any dose-limiting toxicities and a good safety profile. Encouraging clinical activity was observed in the hematologic cancer cohort with a 30% DCR on the 10 patients evaluable at week 8 or beyond (Garciaz, SITC 2023) supporting further clinical testing of ICT01 in combination with Venetoclax (VEN) and 5-Azacytidine (AZA), considered the standard of care for patients with newly diagnosed AML who are not candidates for intensive chemotherapy. Both ICT01 + γ9δ2 T cells and VEN/AZA induce killing of AML blasts, while VEN/AZA has been shown to enhance T-cell- and NK-cell-mediated cytotoxicity against AML blasts, suggesting that the combination of ICT01 plus VEN/AZA could lead to greater efficacy. We used preclinical models to demonstrate the ability of VEN/AZA to potentiate the anti-leukemic activity of ICT01-activated γ9δ2 T cells to support the clinical evaluation of ICT01 + VEN/AZA in 1L AML patients eligible to receive VEN/AZA.
Results:
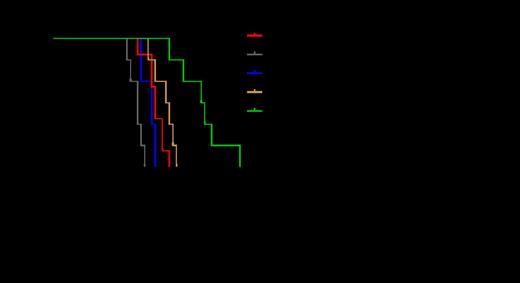
Preclinical in vitro studies showed that ICT01-mediated activation of g9d2 T cells significantly increased survival against VEN toxicity by >50%, as compared to untreated control (p<0.01). Furthermore, killing assays performed by co-culture of AML blasts and HD-PBMC demonstrated that VEN/AZA enhances the blast killing capacity of ICT01-activated g9d2 T cells. These results were confirmed in vivo in NSG mice engrafted with human AML that were adoptively transferred with human g9d2 T cells and treated with VEN (40mg/kg/day OG)/AZA (2mg/kg/day IP), ICT01 (1mg/kg IV) or the combination. In this model the use of ICT01 together with VEN/AZA significantly delayed tumor growth (p<0.001) and significantly prolonged median survival to 42.5 days (vs 28 and 32.5 days for animals receiving ICT01 or VEN/AZA, respectively) (Figure 1).
These results led to the design of the recently initiated Phase 2a arm of EVICTION in 1L AML where ICT01 is given at 10 mg or 75 mg IV Q4W on Day 1 of the 28-day approved regimen of VEN/AZA until toxicity or disease progression. Up to 25 patients will be treated at each dose level, following an interim analysis after 10 pts are treated at each dose. Clinical sites in France, Spain and the USA are participating. Biomarkers include multiparametric flow cytometry analysis of g9d2 T cells and other immune cells, and BTN3A expression in peripheral blood and in bone marrow aspirates at baseline and on Day 21-28. Circulating cytokine levels (including IFNγ, TNFa, IL-1ß, IL-2, IL-4, IL-6, IL-8, IL-10, IL-17a and MCP-1) are also assessed with MSD technology. Clinical response is evaluated per AML-specific standards (e.g., Cheson/IWG Criteria), with a target CR rate of >40%. Preliminary safety, biomarker and efficacy data from patients will be presented.
Conclusion:
Encouraging results obtained in the EVICTION Phase 1 trial and preclinical demonstration of the benefit of using ICT01 plus VEN/AZA allowed the initiation of a Phase 2a expansion cohort to evaluate the clinical benefit of this combination in 1L AML patients.
Disclosures
Maiti:Celgene: Research Funding; Lin BioScience: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal